Multiple choice: Select on or more answers and click on them to see if they are correct.
Fill in the gaps. Toggle down to check your results.
1. Molecules possess a specific hydrophobicity/ hydrophilicity, which is expressed as logP or logD value. At pH 7.4 the following molecules have a specific logD value. Find matches between the logD values below and the molecules.
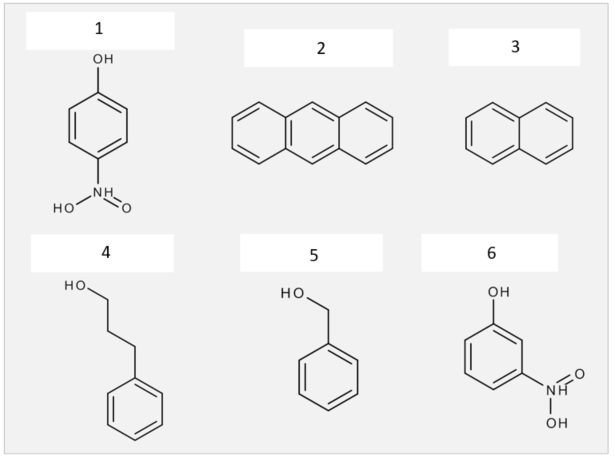
logD values: a) 1.94, b) 0.59, c) 2.96, d) 3.95, e) – 3.40, f) -3.40
Solution
1e, 2d, 3c, 4a, 5b, 6f
2. Match the numbers in the brackets with the words below.
Polarity describes (1) within a molecule, which depends on (2) of its contained (3).
a) atoms, b) electrons, c) the electric charge distribution, d) the electrical conductivity, e) electronegativity
Solution
1c, 2e, 3a
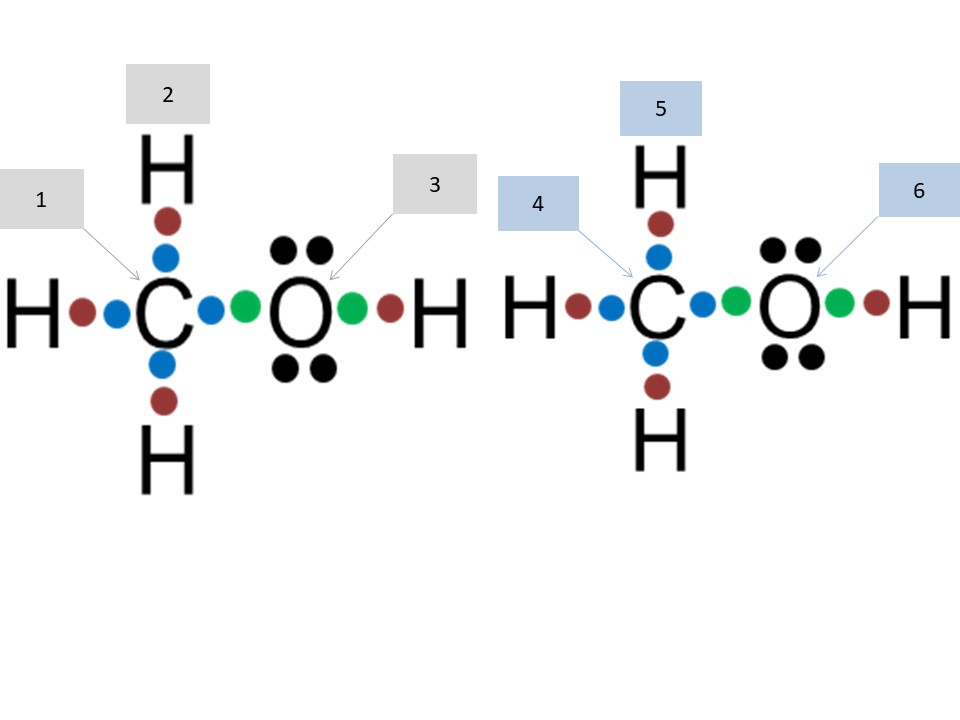
3. Match the provided values of electronegativity and numbers of valence electrons of H, C or O with the numbers in the grey or blue bloxes. Use a periodic table of elements for reference.
a) 2.20, b) 4, c) 2.55, d) 1, e) 3.44, f) 6
Solution
1c, 2a, 3e, 4b, 5d, 6f
4. Match the numbers in brackets with the words below.
Unequal (1) of covalent bond forming atoms results in unequal sharing of (2), which results in (3) and (4) charges on (5) ends of the (6). These bonds are therefore called (7).
a) partially positive, b) neutral, c) polar, d) partially negative, e) hydrophobic, f) same, g) electronegativity, h) opposite, i) electrons, j) protons, k) chemical bond
Solution
1g, 2i, 3a, 4d, 5h, 6k, 7c
5. Label the fields by means of the provided selection. Hint: You may use a period table of elements to look up the electronegativity of H and Cl.
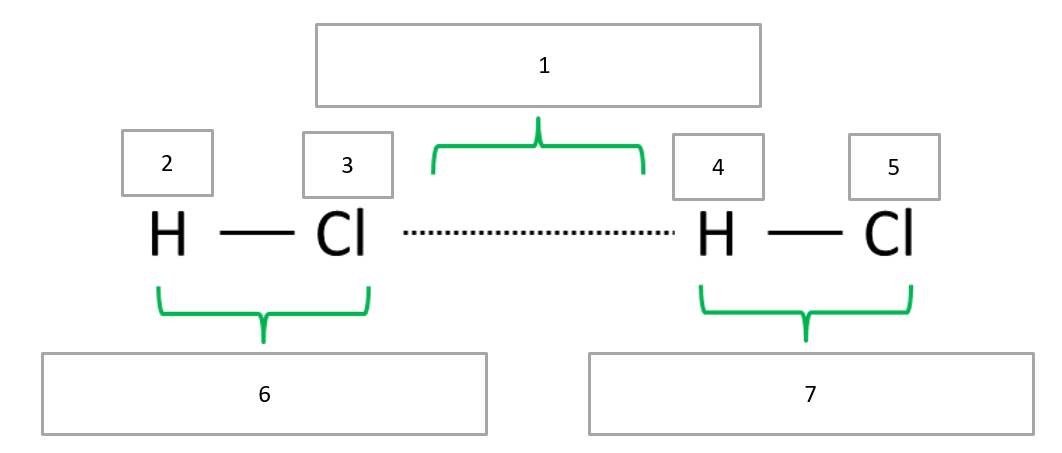
a) δ− (2x), b) dipole-dipole force, c) δ+ (2x), d) permanent dipole (2x)
Solution
1b, 2c, 3a, 4c, 5a, 6d, 7d
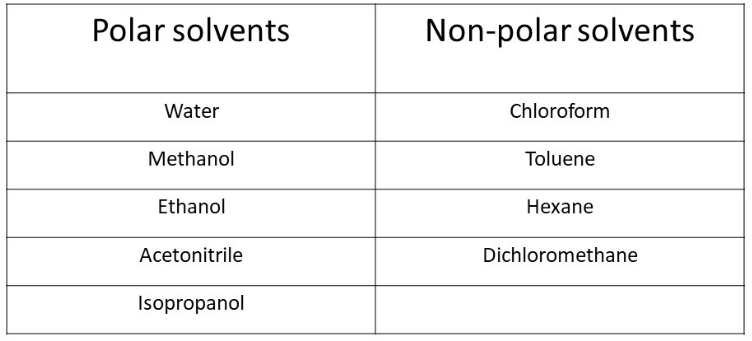
6. Devide whether the given solvents are polar or non-polar. Create a table.
Acetonitrile, dichloromethane, water, ethanol, toluene, hexane, chloroform, isopropanol methanol
Solution
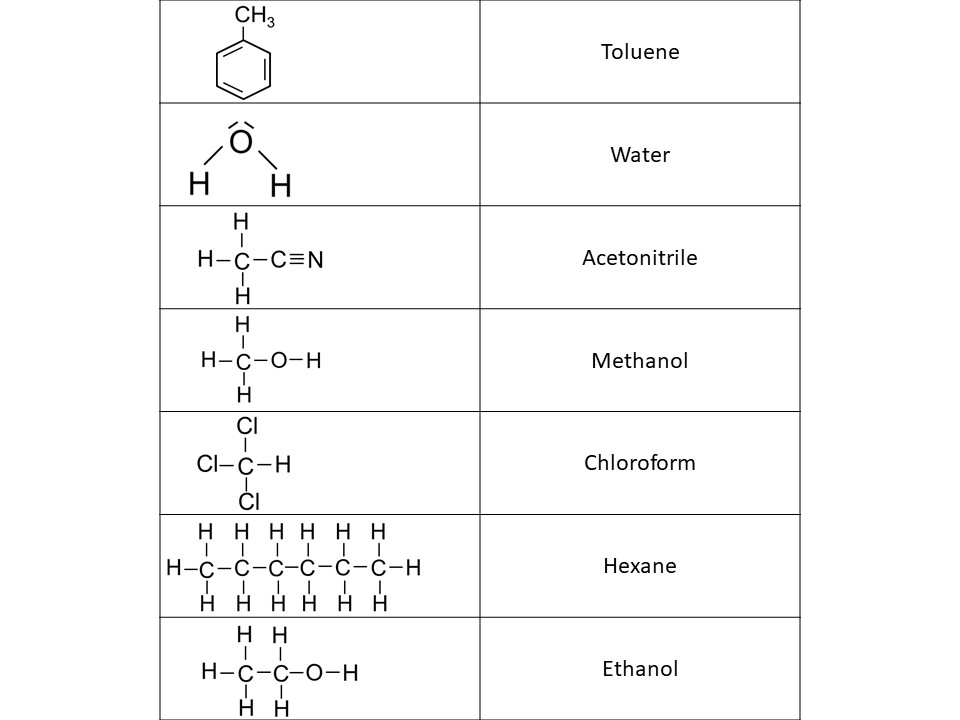
7. Name the structural formulas of polar and non-polar solvents.
Solution
8. Match the numbers in brackets with the words below.
LogD refers to (1) molecules, which contain (2) groups. Hence the molecule´s (3) and thus (4) changes with the (5) of the solution.
a) neutral, b) ionizable, c) hydrophobicity, d) chargeable, e) pH value, f) hydrophilicity, g) temperature, h) solubility
Solution
1b, 2d, 3c, 4h, 5e
