Multiple choice: Check your answers by clicking on them.
1. Select the answers which apply for reversed-phase chromatography.
Heptane is a typical solvent.
Unpolar analytes elute earlier than polar ones.
Octadecyl is a typical stationary phase.
The mobile phase is more polar than the stationary phase.
Retention is based on hydrophobicity.
2. The opposite of normal-phase chromatography is … chromatography.
non-normal phase
stationary
reversed-phase
thin-layer
HILIC
3. Typical examples for reversed-phase stationary phases are:
Amide
Carboxylic acids
Phenyl
C18
C8
4. What is the main characteristic of an isocratic compared to a gradient elution method?
The mobile phase condition starts with a high proportion of organic solvent.
Isocratic elution is more powerful than gradient elution.
The mobile phase composition remains constant.
The organic solvent proportion is more rapidly increased.
5. In normal-phase chromatography the mobile phase is … than the stationary phase.
equally polar as
more polar than
more non-polar than
6. The normal phase retention mechanism is based on … of either the mobile phase molecule or the analyte onto the stationary phase.
mobile phase repulsion
solvent viscosity
polar absorption
pH distribution
non-polar interaction
7. What are the effects of a moderately elevated temperature (e.g. 20 to 50 °C) on a chromatographic separation?
Stationary phase degenerates
Peak tailing
Increased retention times
Shorter retention times
Succession of analyte elution can be altered
8. Choose those characteristics which apply for isocratic elution.
Solvent composition is constant
No equilibration necessary
Early peaks are well separated
Long analysis time
Solvent composition changes over time
9. Increasing column length results in…
… lower plate number
… increased analysis time
… narrower peaks
… higher efficiency
10. In reversed-phase chromatography the stationary is … the mobile phase.
more polar than
more non-polar than
more soluble than
equally polar as
Fill in the gap: Toggle down to check your results.
1. Sort the stationary phases by hydrophobicity (1 = highest hydrophobicity):
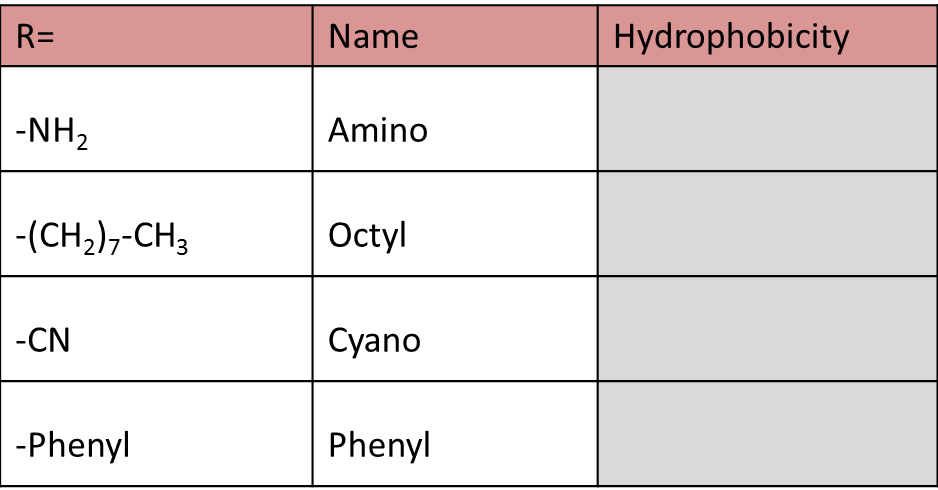
Solution
2. Match the numbers in the boxes with the words below.
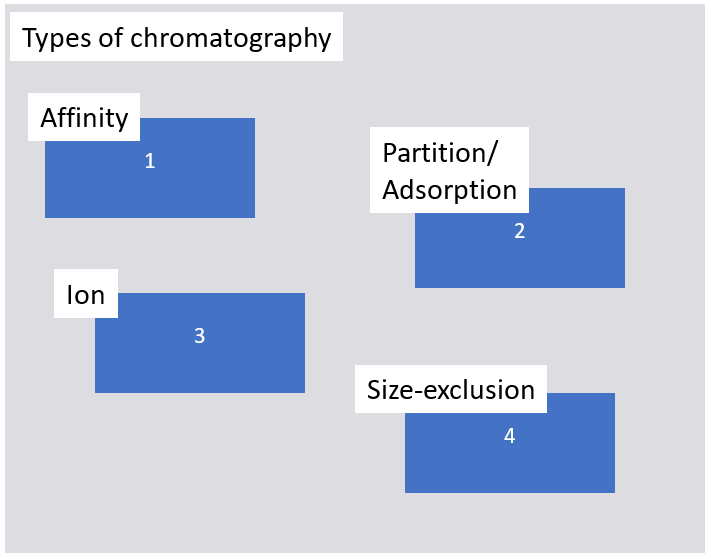
a) biological interaction, b) hydrophobicity, c) size, d) charge
Solution
1a, 2b, 3d, 4c
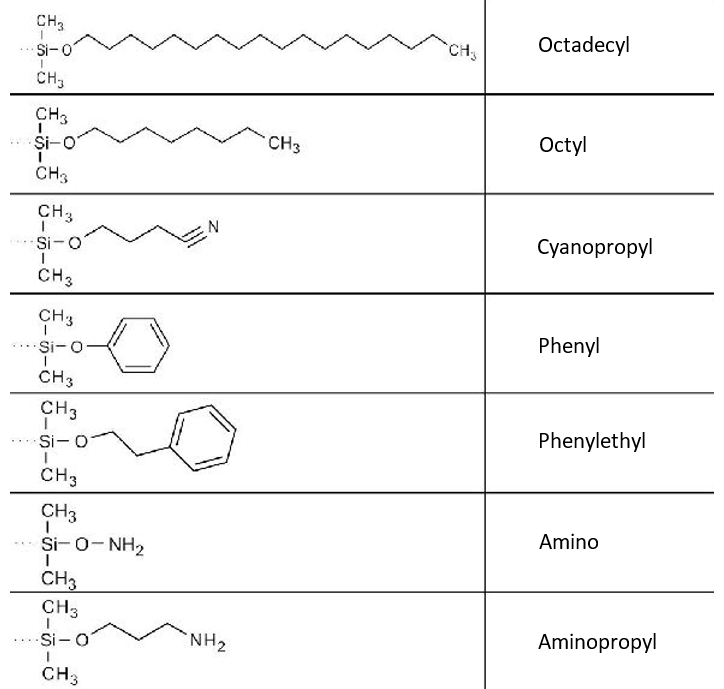
3. Name the stationary phases shown below.
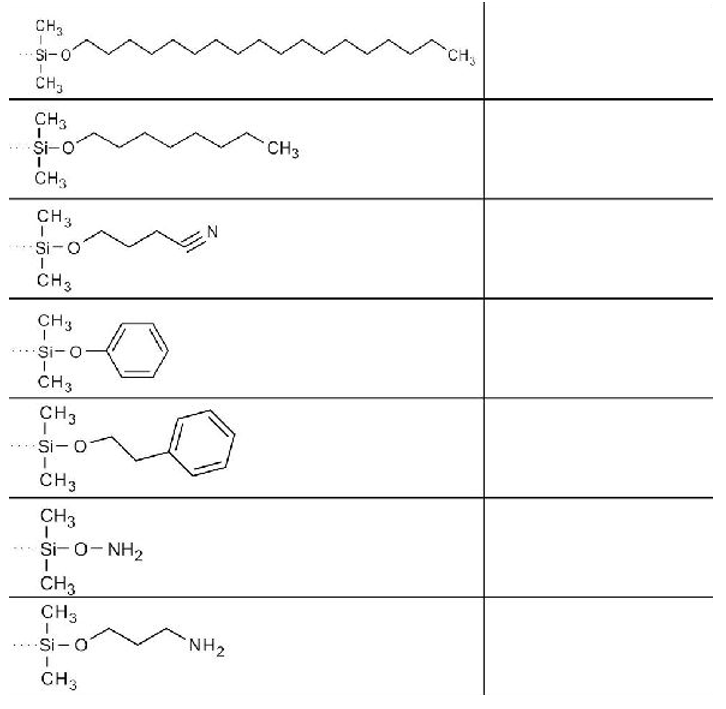
Solution
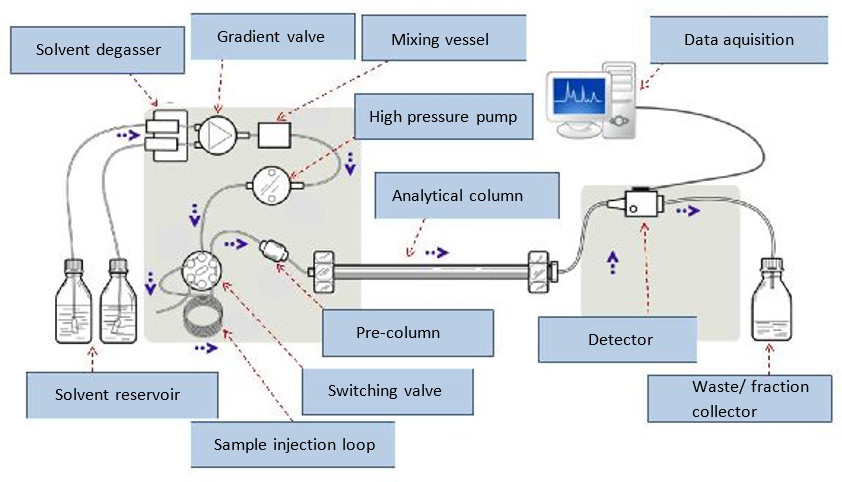
4. Label the HPLC parts.
Solution
5. Match the numbers in the boxes with the words below.
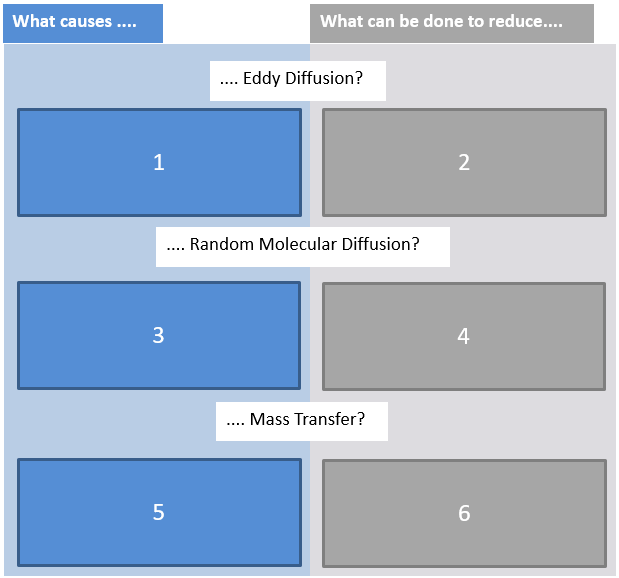
a) inhomogeneous packing material, b) movement alongside the column, c) small stationary phase particles, d) particles with narrow size distribution, e) high mobile flow rates, f) short and narrow tubings, g) entering of analyte into pores, h) low mobile flow rates, i) increased temperature
Solution
1a, 2cd, 3b, 4ef, 5g, 6hi
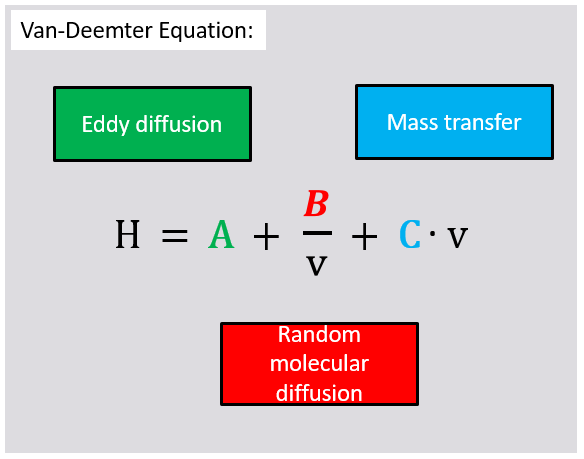
6. Find the correct names for the different terms of the Van Deemter equation.
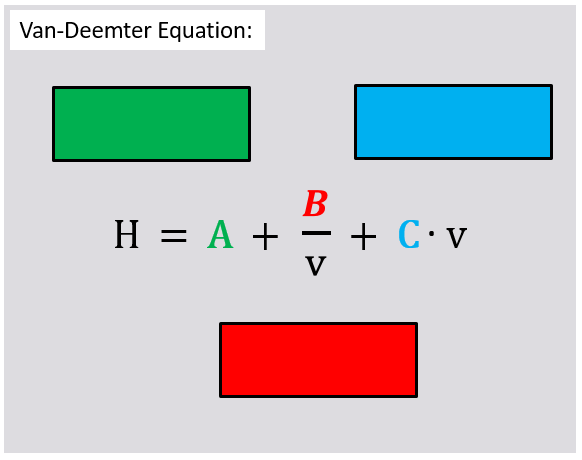
Solution
7. Match the numbers in the brackets with the words below.
In reversed phase chromatography the mobile phase is (1) the stationary phase. (2) in reversed phase chromatography usually consist of water or (3) and an (4). (5) is based on the hydrophobicity of an analyte molecule, which can be expressed as (6). The retention time is (amongst others) influenced by (7) strength of the organic modifier and (8).
a) buffer, b) the retention time, c) mobile phase, d) organic modifier, e) logP, f) the temperature, g) more polar than, h) the solvent
Solution
1g, 2c, 3a, 4d, 5b, 6e, 7h, 8f
7. Rank the following compounds according to their adsorption ability in a decreasing order.
Amides, saturated hyrocabons, olefins, aldehydes, aromatics, nitro compounds, carboxylic acids, ketones, alcohols, amines, esters
Solution
[carboxylic acids] > [amides] > [amines] > [alcohols] > [ketones] > [aldehydes] > [esters] > [nitro compounds] > [aromatics] > [olefins] > [saturated hyrocabons]










