Browse the glossary using the index:
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
2D Electrophoresis
2D electrophoresis involves two independent electrophoretic steps. Proteins are initially separated via isoelectric focussing, followed by a further gel electrophoresis step (e.g. SDS Page).
Adsorption Chromatography
Separation is based mainly on differences between the adsorption affinities of the sample components for the surface of an active solid.
Affinity Chromatography
This expression characterizes the particular variant of chromatography in which the unique biological specificity of the analyte and ligand interaction is utilized for the separation.
Chemical bond
- non-polar covalent bond (e.g. methane) → similar electronegativity
- polar covalent bond (e.g. methanol) → different electronegativity → induction of a dipole
- ionic bond (e.g. NaCl) → attraction between positively and negatively charged ions → distinctly different electronegativity
Chromatography
Chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction.
Column
The tube and the stationary phase contained within, through which the mobile phase passes.
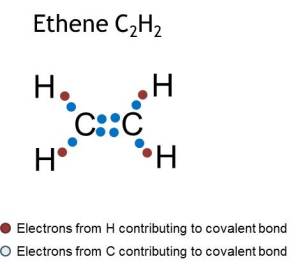
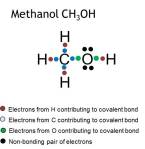
Covalent bond
Covalent bonds between two atoms consist of a shared pair(s) of electrons.
Critical micellar concentration
Concentration of a detergents above which the detergent forms micelles.
Detergent
Detergents are amphiphil molecules, i.e. consisting of a hydrophilic and a hydrophobic part. They are able to align so as to form micelles with the hydrophobic part of the detergent orientated to the inside of the micelle to avoid interaction with water.
They are e.g. used for the solubilization of hydrophobic compounds, the denaturation of proteins or the isolation of transmembrane proteins (which contain hydrophobic areas physiologically located within the cell membrane).
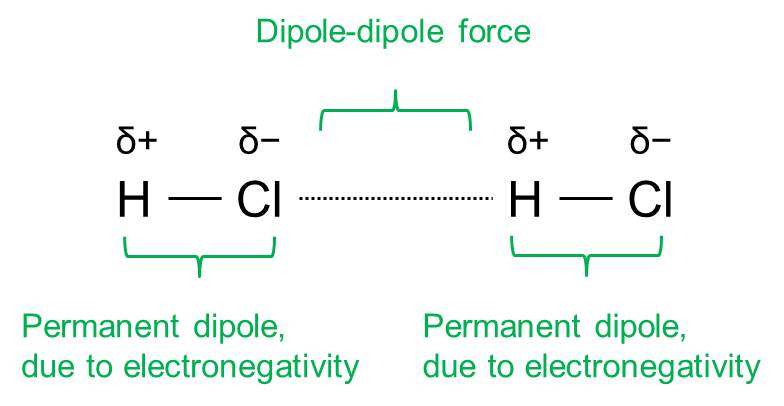
Dipole-dipole interaction
Electrostatic force between two permanent dipoles. Dipoles are induced by distinct different electronegativies of atoms.
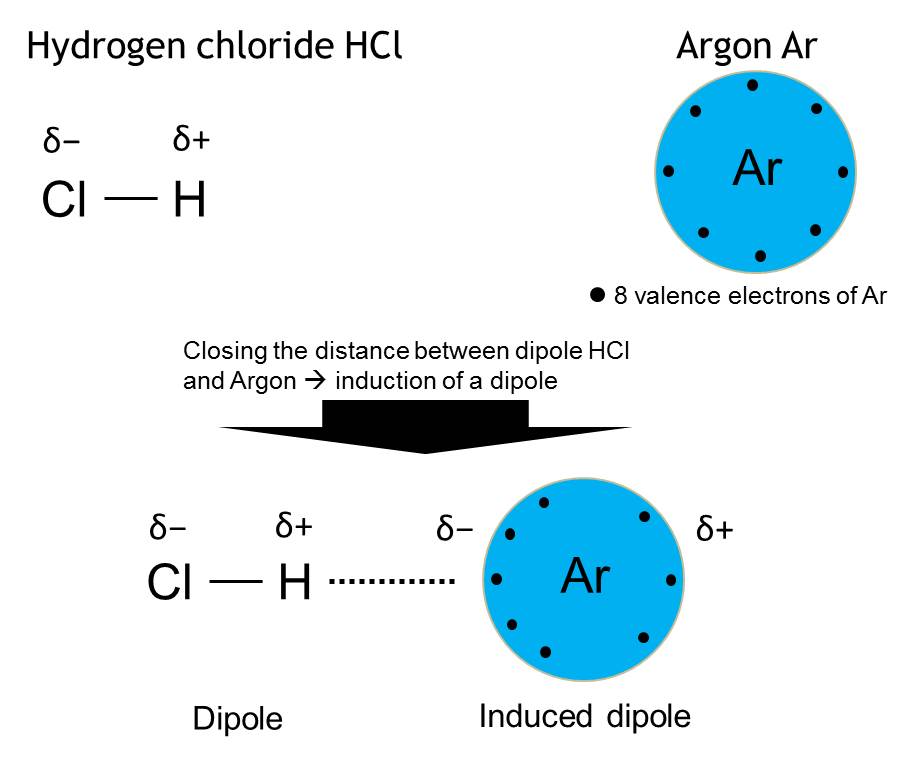
Dipole-induced dipole interaction
Interaction of a permanent dipole and a non-polar molecule or atom, that induces a dipole in the non-polar molecules/atom by attracting its electrons.
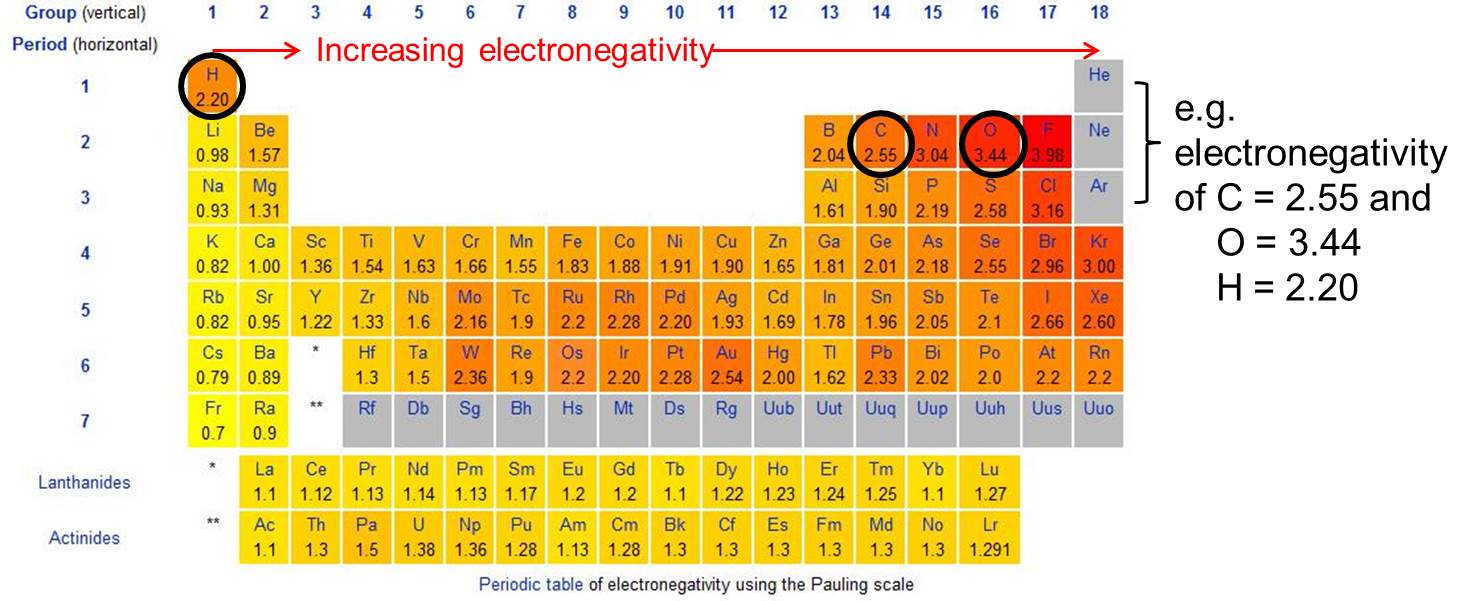
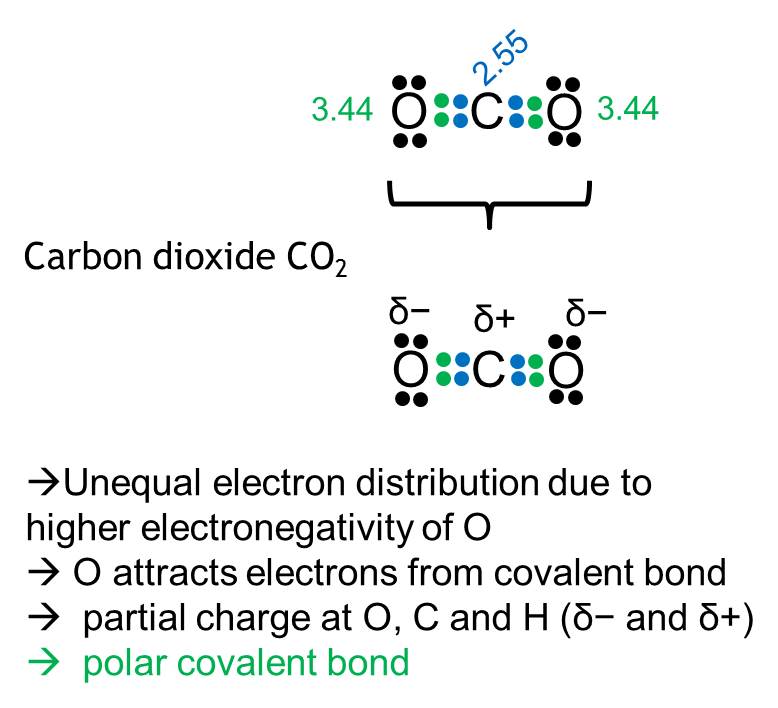
Electronegativity
Ability of an atom or functional group to attract electrons towards itself.
Elution
The process of passing mobile phase through the column to transport solutes down the column.
Gas Chromatography
A separation technique in which the mobile phase is a gas. Gas chromatography is always carried out in a column.
Gradient Elution
The procedure in which the composition of the mobile phase is changed continuously or stepwise during the elution process.
Homogenization
Sample disruption and therewith homogenization, e.g via mechanical methods like milling or ultrasonic. With homogenization the sample should change its physical properties and simultaneously maintaining its overall chemical composition.
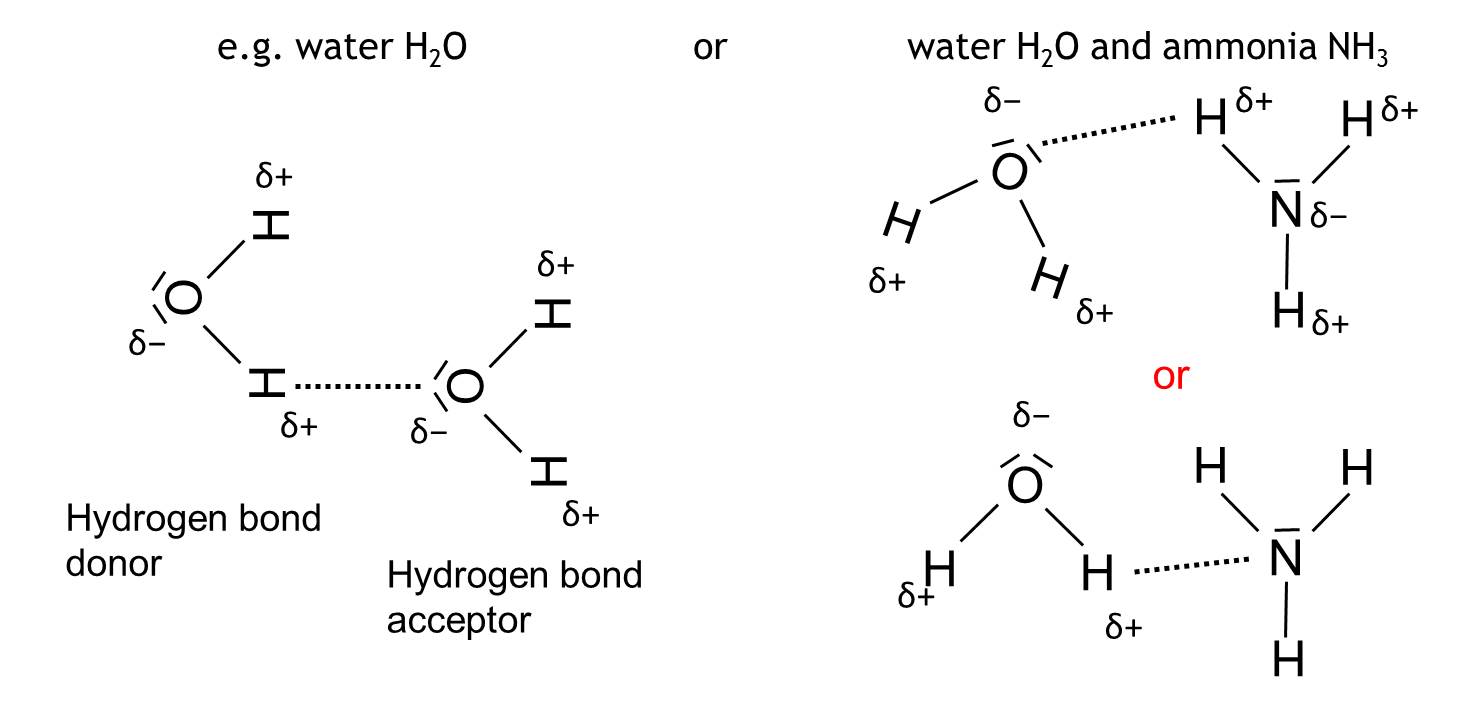
Hydrogen bond
Interaction between two polar molecules between a hydrogen and a electronegative atom like O, N or F.
Intermolecular force
- Forces between two permanent dipoles are called dipole-dipole interactions
- A dipole can interact with a positively or negatively charged ion (e.g. NaCl in water)
- Permanent dipoles have the capability to induce dipoles in neutral molecules or atoms
Intramolecular forces
- Non-polar covalent bond (identical or highly similar electronegativity)
- Polar covalent bonds (medium difference of electronegativity)
- Ionic bonds (distinct difference of electronegativity results in migration of electron(s) and formation of positively and negatively charged ions)
→ Ion are atoms that has lost or gained electron(s)
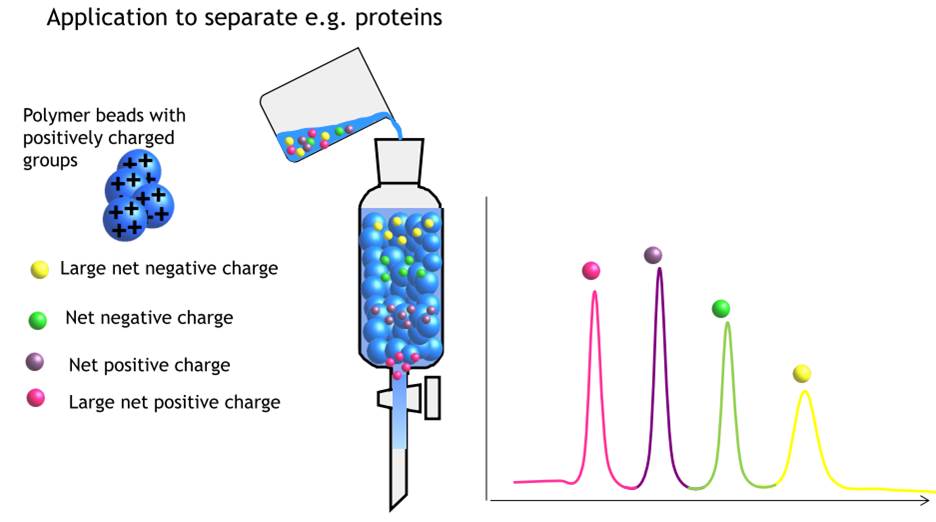
Ion exchange chromatography
Ion exchange chromatography allows the separation of ions and polar molecules. It can be used for all kind of charged molecule, e.g. proteins, amino acids.
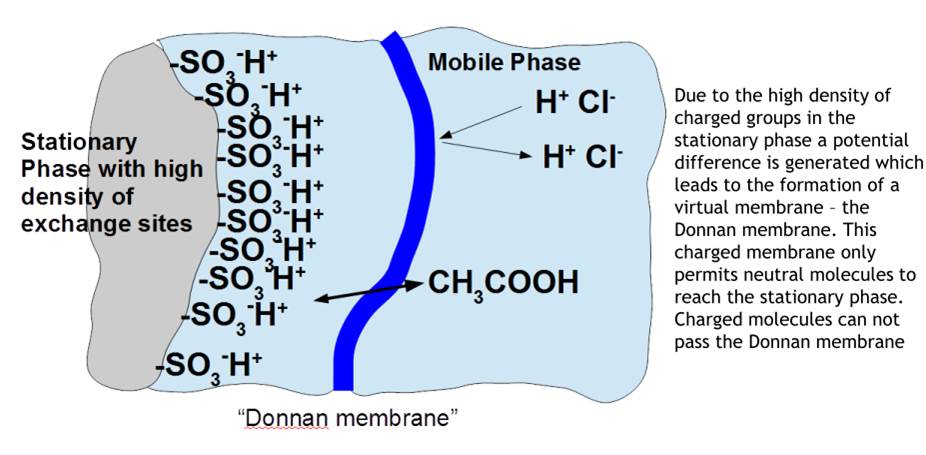
Ion exclusion chromatography
Separation of ionic solutes via exclusion effects, e.g. differences in molecular size, shape and charge.
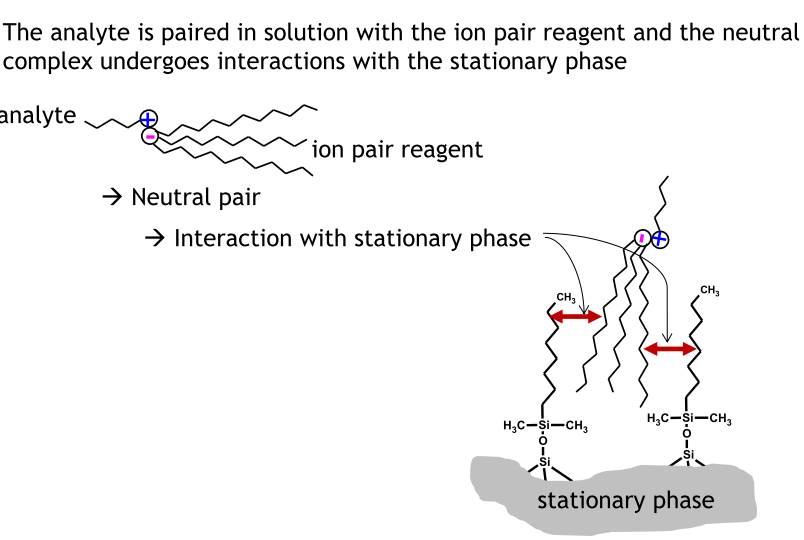
Ion pair chromatography
- Used to separate ionic analytes on a reversed phase column
- For substances which are charged too strongly for adsorption chromatography and are not water soluble enough for ion chromatography
- An organic salt containing a large organic counterion is added to the mobile phase as an ion pairing reagent
- Typical ion pair reagents are: alkylsulphonates, alkylsulphates, tetraalkylammonium ions etc.
Ion-dipole interaction
Electrostatic force between an ion (e.g. Na+ or Cl–) and a dipole (e.g. water).
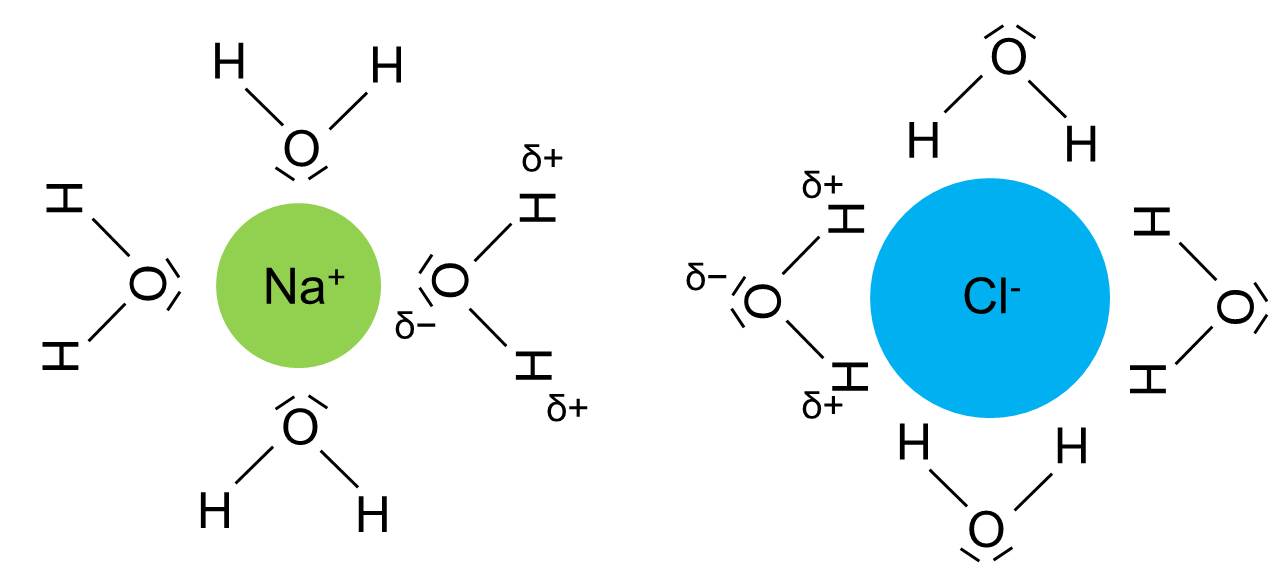
Ionic bond
Bond or electrostatic attraction between a positively and negatively charged ion.
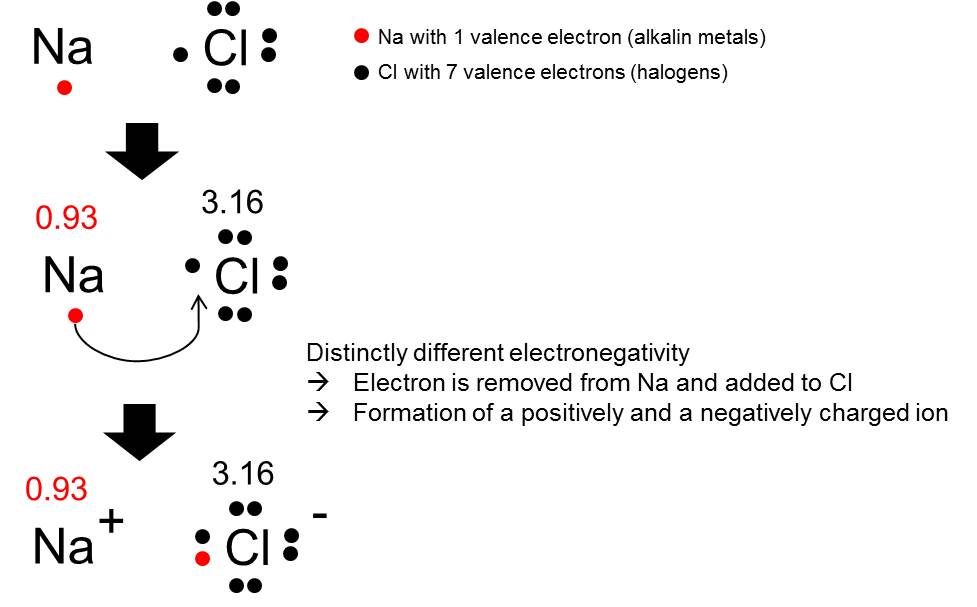
Isocratic Elution
The procedure in which the composition of the mobile phase remains constant during the elution process.
Isoelectric focussing
Depending on the proteins amino acid composition, they carry a specific surface charge, which is affected by changes in pH value. Using a strip gel electrophoresis with pH gradient, proteins can be separated according to their isoelectric point at which they carry a zero net charge.
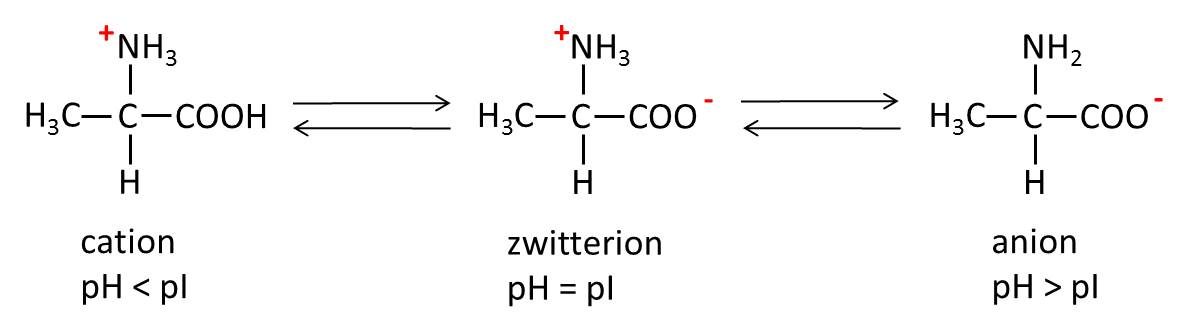
Isoelectric point
pI is the pH at which a molecule carries no net electrical charge. At a pH below the pI, the molecule will carry a positive charge. At a pH above the pI, it will carry a negative charge (e.g. amino acid alanine (pI = 6.0) (compare figure)).
Liquid Chromatography
A separation technique in which the mobile phase is a liquid. Liquid chromatography can be carried out either in a column or on a plane.
Note: Present-day liquid chromatography generally utilizing very small particles and a relatively high inlet pressure is often characterized by the term High-Performance (or High-pressure) Liquid Chromatography, and the acronym HPLC.
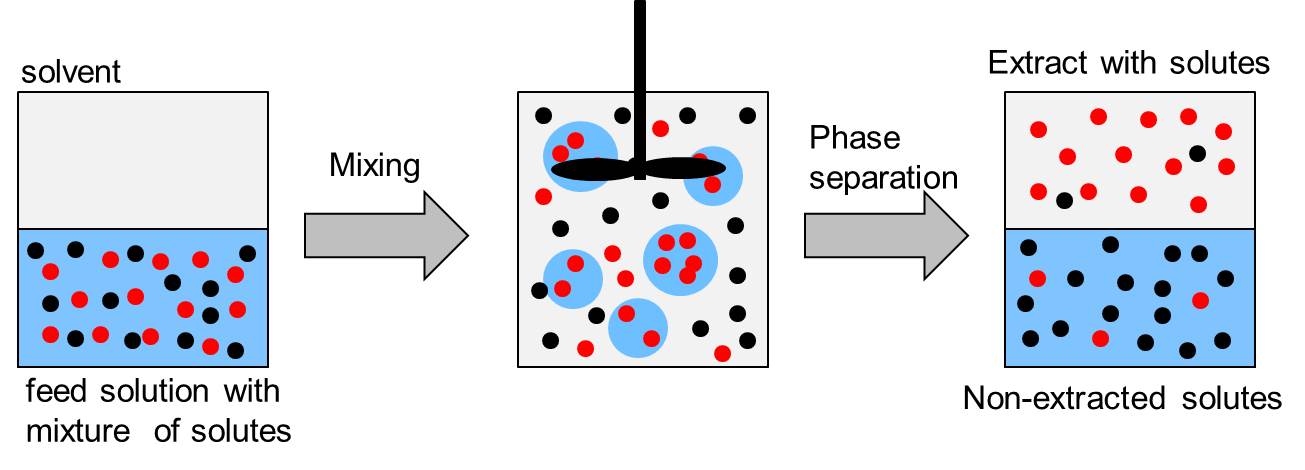
Liquid-liquid extraction
Transfer of compounds from one solution (feed solution) to another solvent, which is immiscible with the feed solution. This method separates compounds based in their solubility in two different immiscible liquids.
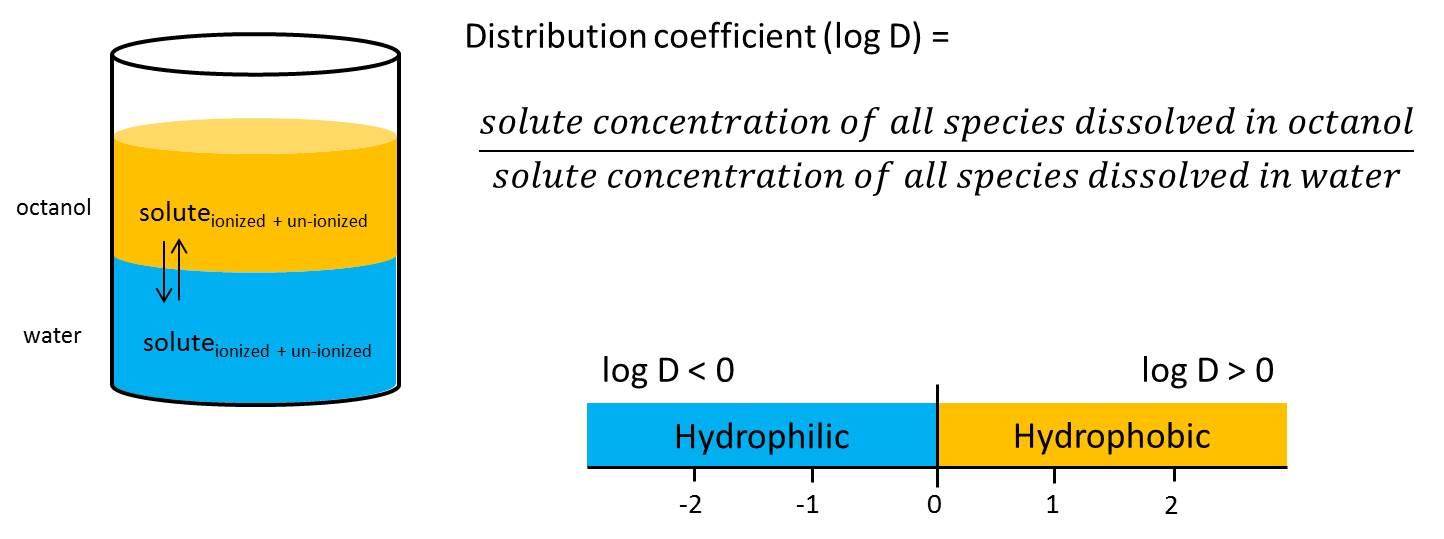
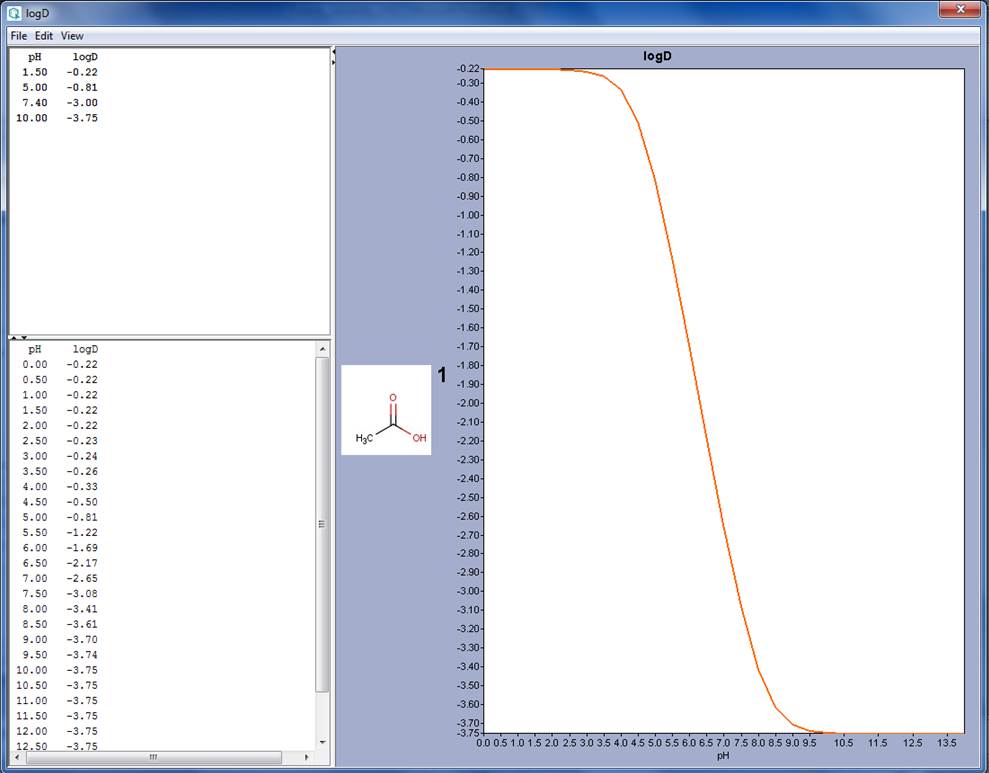
logD
= Distribution coefficient
- log D refers to ionizable molecules lLipophilicity and therefore solubility changes as a function of pH for ionisable compounds).
- pH dependence of logD (e.g. acetic acid → compare figure created with MarvinSketch 14.8.25.0, ChemAxon)
- Increasing pH results in ….
…. increasing abundance of negativelycharged acetic acid molecules
…. decreasing logD (-0.22 at pH 1.5 to -3.75 at pH 10)
…. increasing solubility of acetic acid in aqueous solution
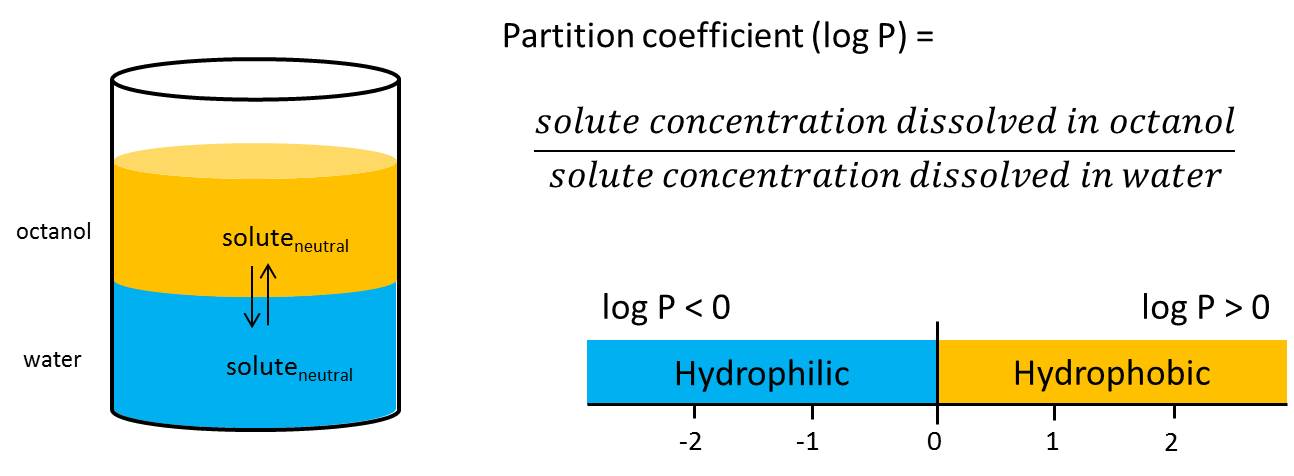
logP
= Partition coefficient
log P refers to neutral, non-ionizable molecule species. Lipophilicity/ hydrophilicity of a molecules is determined according to its distribution in a biphasic system, consisting of two immiscible solvents (e.g. octanol and water
Lyophilization
Lyophilization is a dehydration process (removal of water) to preserve material, e.g. food. At first the material is frozen, followed by the reduction of the surrounding pressure.The frozen water in the sample is sublimated (direct transfer from solid to gas phase).
Mass spectrometry
A mass spectrometer measures the abundance and mass-to charge ratio (m/z) of ions. An ion is an atom or molecule with a higher or lower number of electrons compared to its number of protons. The atom or molecule possesses a positive (less electrons than protons) or negative (more electrons than protons) net charge.
Mobile Phase
A fluid which percolates through or along the stationary bed, in a definite direction. It may be a liquid (Liquid Chromatography) or a gas GasChromatography) or a supercritical fluid (Supercritical-Fluid Chromatography).
Native Page
Gel electrophoretic separation of non-degraded proteins in their native conformation. The proteins are separated according to their charge, mass and spacial extent.
Normal Phase Chromatography
An elution procedure used in liquid chromatography in which the stationary phase is more polar than the mobile phase. This
term is used in liquid chromatography to emphasize the contrast to Reversed Phase Chromatography.
Northern Blot
Northern blot method is employed for the gel electrophoretic separation of RNA, which is then blotted onto a membrane (compare also „Western blot“). The target RNA is detected by adding specific RNA probes, which bind to the target RNA. Those probes are either labelled with radioisotopes or coupled to a reporter enzyme, whereby a detectable signal is generated by means of enzymatic substrate degradation.
Partition Chromatography
Separation is based mainly on differences between the solubilities of the sample components in the stationary phase (gas chromatography), or on differences between the solubilities of the components in the mobile and stationsuy phases (liquid chromatography).
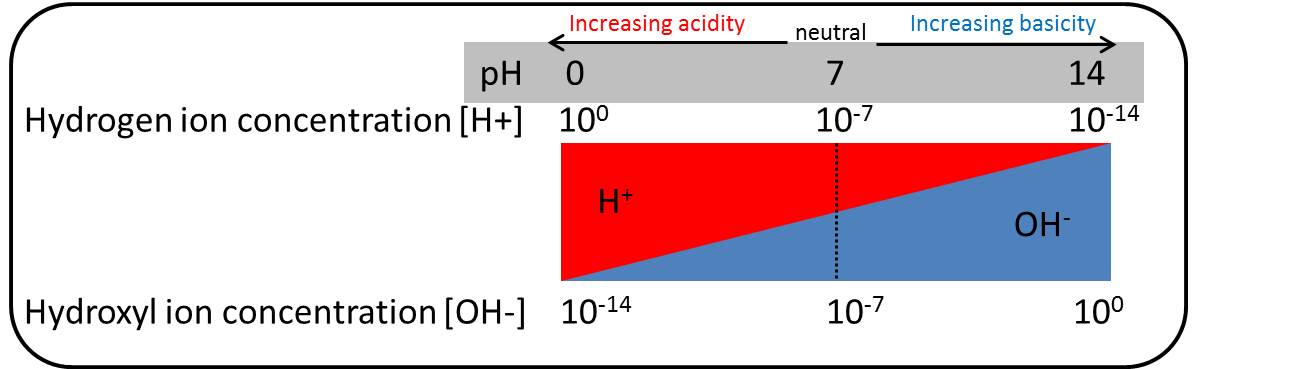
pH
pH reflects the concentration of hydrogen ions (H+)/hydroxyl ions (OH-) in the solution. The proton (i.e. H+) concentration increases with decreasing pH.
pKa (acid dissociation constant)
pKa (acid dissociation constant)
pKa is the pH at which the abundance of ionized and non-ionized from is equal e.g. acetic acid (CH3COOH) with a pKa of 4.8.
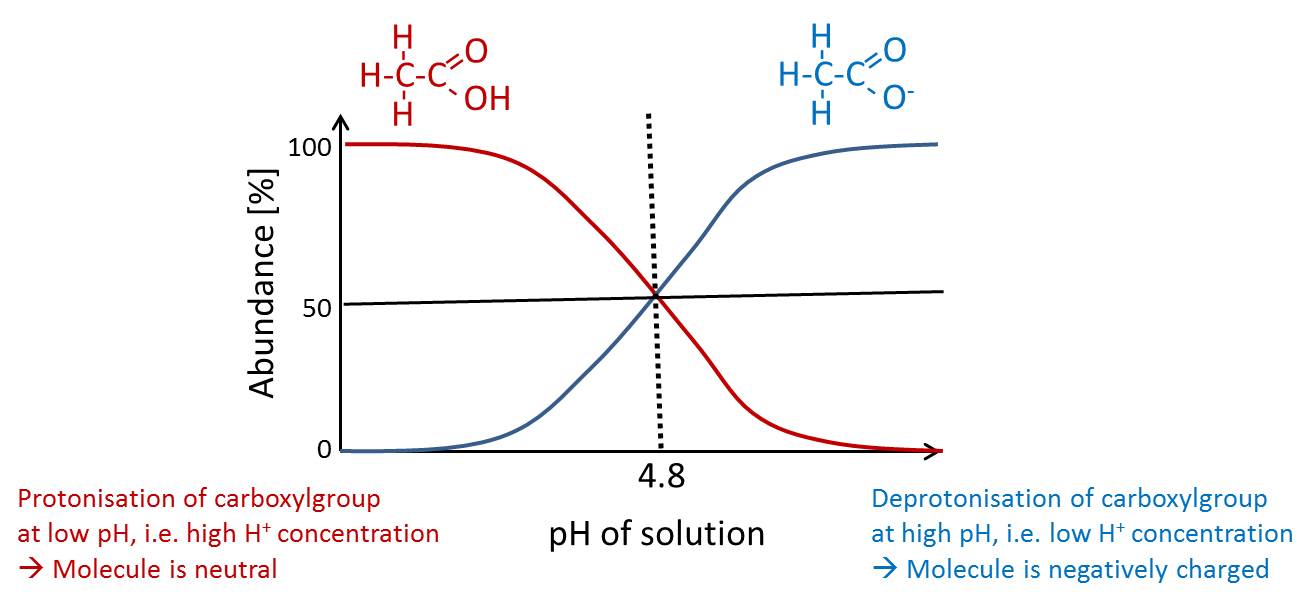
Polarity
Polarity describes the electric charge distribution within a molecules, which is dependent on the electronegativity of its containing atoms. Polar molecules can form e.g. hydrogen bonds. (compare slides „Polarity Basics“)
Reversed Phase Chromatography
An elution procedure used in liquid chromatography in which the mobile phase is significantly more polar then the stationary phase, e.g., a microporous silica-based material with chemically bonded alkyl chains.
Note: The term „reverse phase“ is an incorrect expression to be avoided.
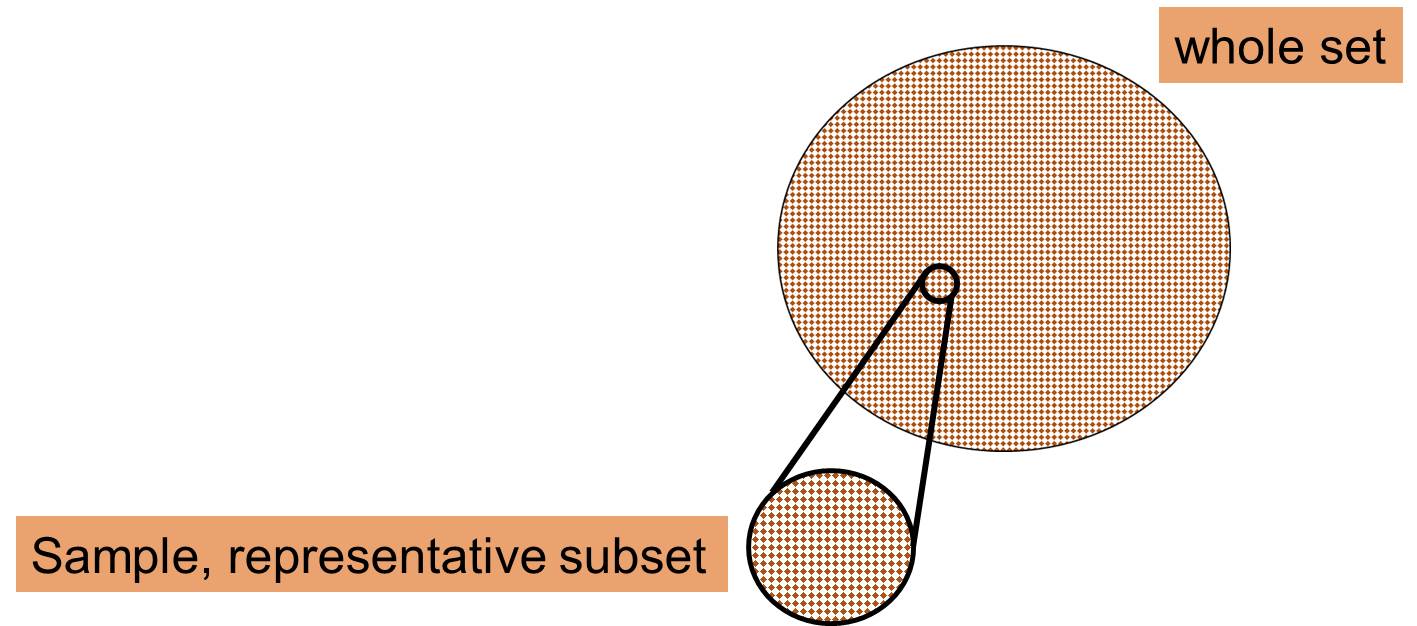
Sample
- A small part which is collected in order to represent the whole.
- A subset of managable size of a population (representative subset).
- Collected and selected by a defined procedure.
- Sample is taken in order to identify patterns and trends.
SDS Page
Gel electrophoretic separation of denatured proteins. Denaturation and separation is achieved through the addition of the negatively charged detergent SDS.
Size Exclusion Chromatography
A mode of LC in which substances are separated by virtue of their size in solution. Also known as gel permeation chromatography, gel filtration chromatography or gel chromatography.
Solid phase extraction
Solutes contained in a sample are separated from from each other according to their chemical and physical properties. Samples can be purified and concentrated or an analyte of interest can be isolated.
The analyte remains on the column (high affinity to stationary phase), whereas contaminants pass through, are collected and discarded. The analyte can then be eluted by washing the column with an appropriate solvent, in which the analyte will dissolve.
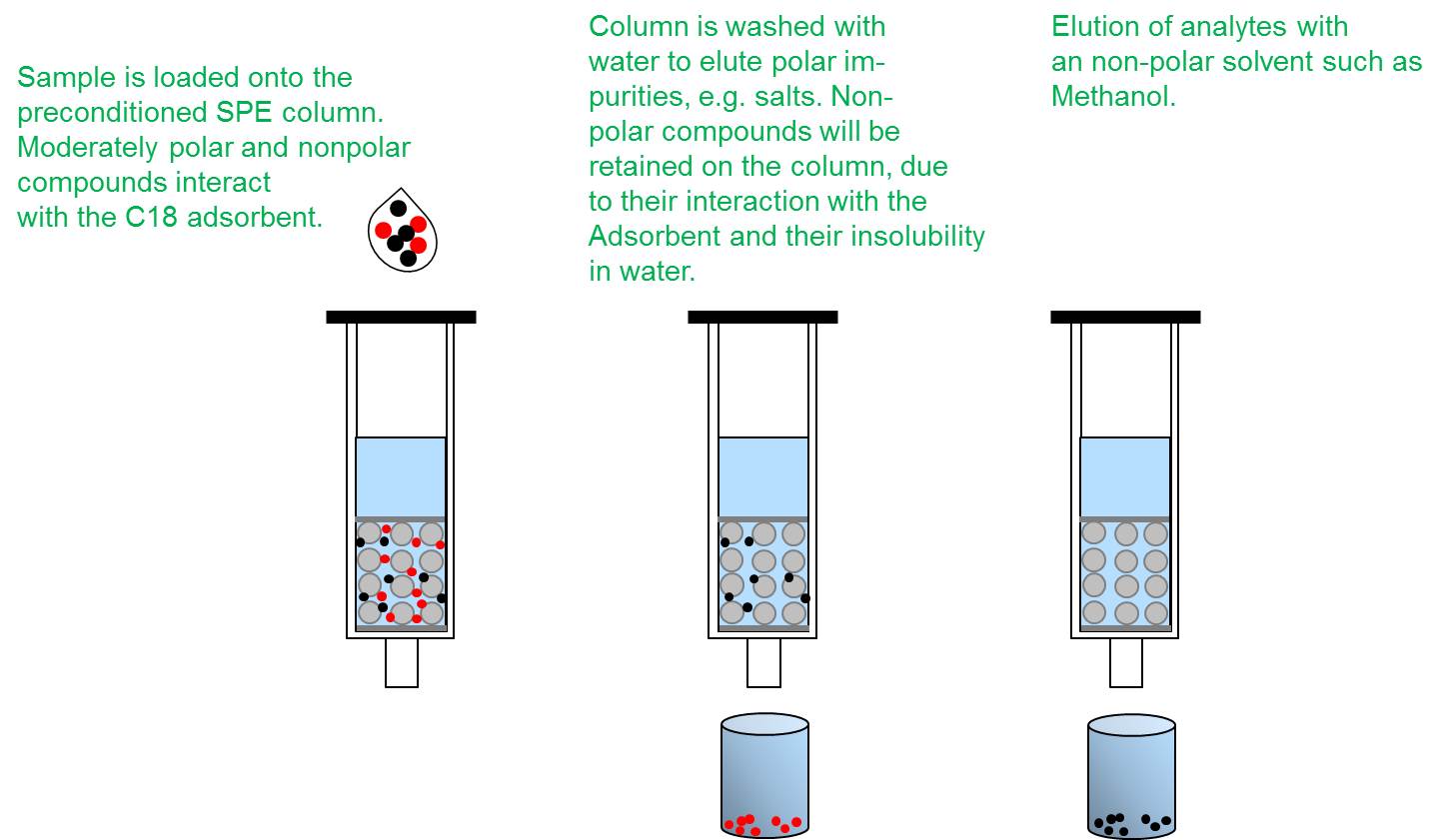
Solubility
The solubility of a solute (= solved compound) highly depends on its properties (e.g. its polarity) and the properties of the solvent as well as on the temperature, the pressure and the pH („Like dissolves like“). A substance can be solved up to a specific and solute- and solvent dependent maximum concentration (=saturation concentration).
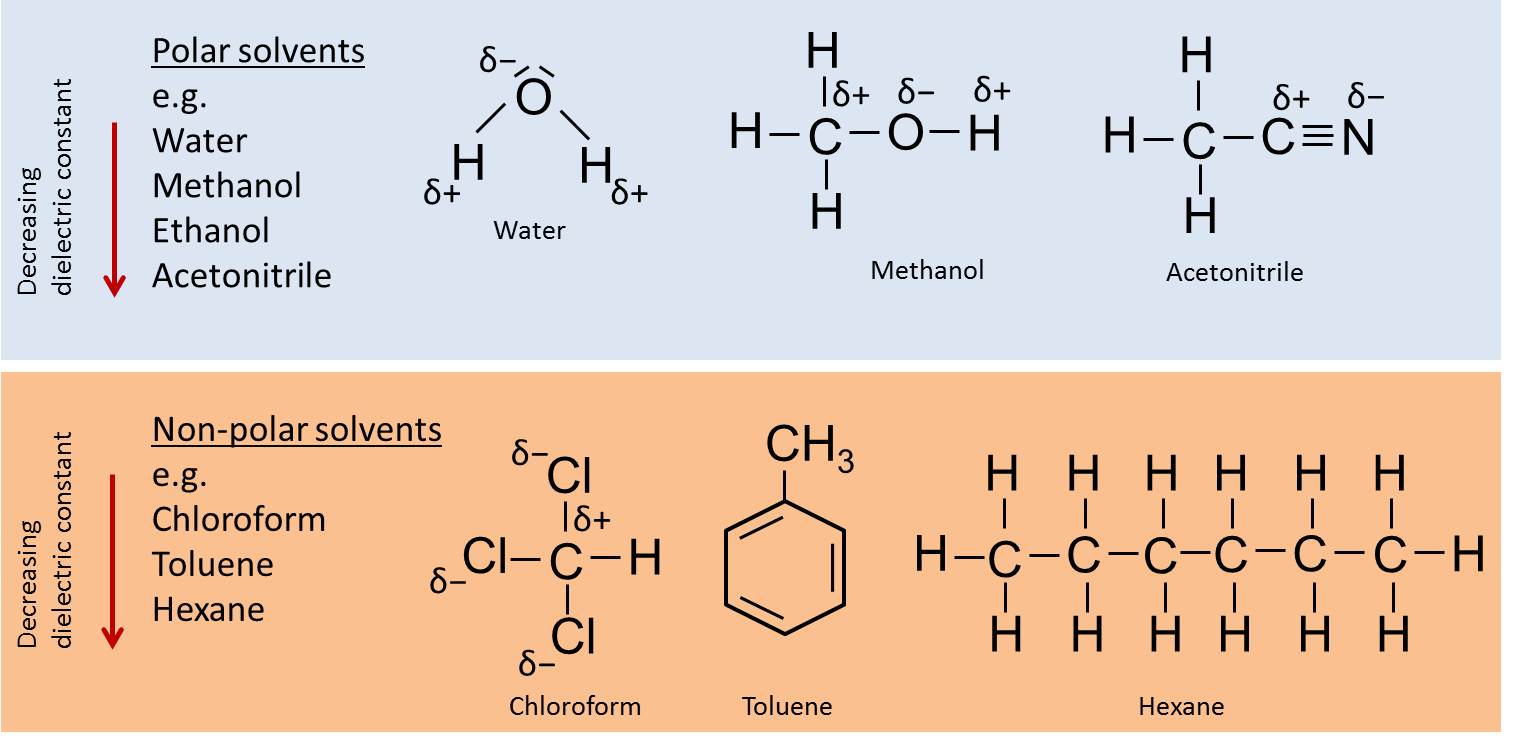
Solvent strength
The elutropic series of solvents reflects the ability of a solvent to elute various solutes from a given sorbent („eluting power“/„solvent strength“) (compare slides „Chromatography“). Although the eluting power depends on the stationary phase as well as on the compounds, the order is usually from polar solvents (e.g. water or methanol) to non-polar solvents (e.g. hexane).
Southern Blot
Southern Blot is employed for the gel electrophoretic separation of DNA. For this purpose the DNA is cutted into small fragments, which are then separated. The next steps involves the blotting of separated DNA fragments to a membrane. Labelled probes, which bind specific DNA target sequences, are added to be able to detect the respective DNA fragments of interest.
Stationary Phase
The stationary phase is one of the two phases forming a chromatographic system. It may be a solid, a gel or a liquid. If a liquid, it may be distributed on a solid.
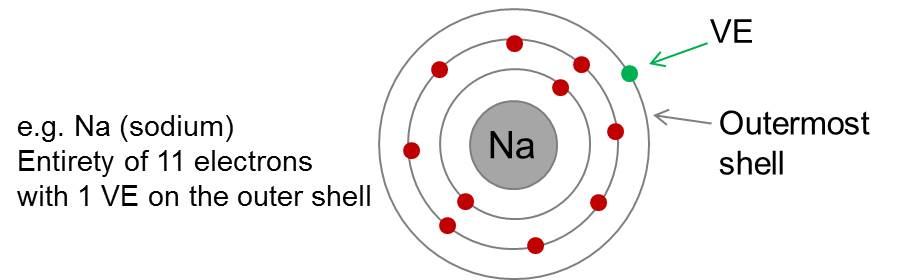
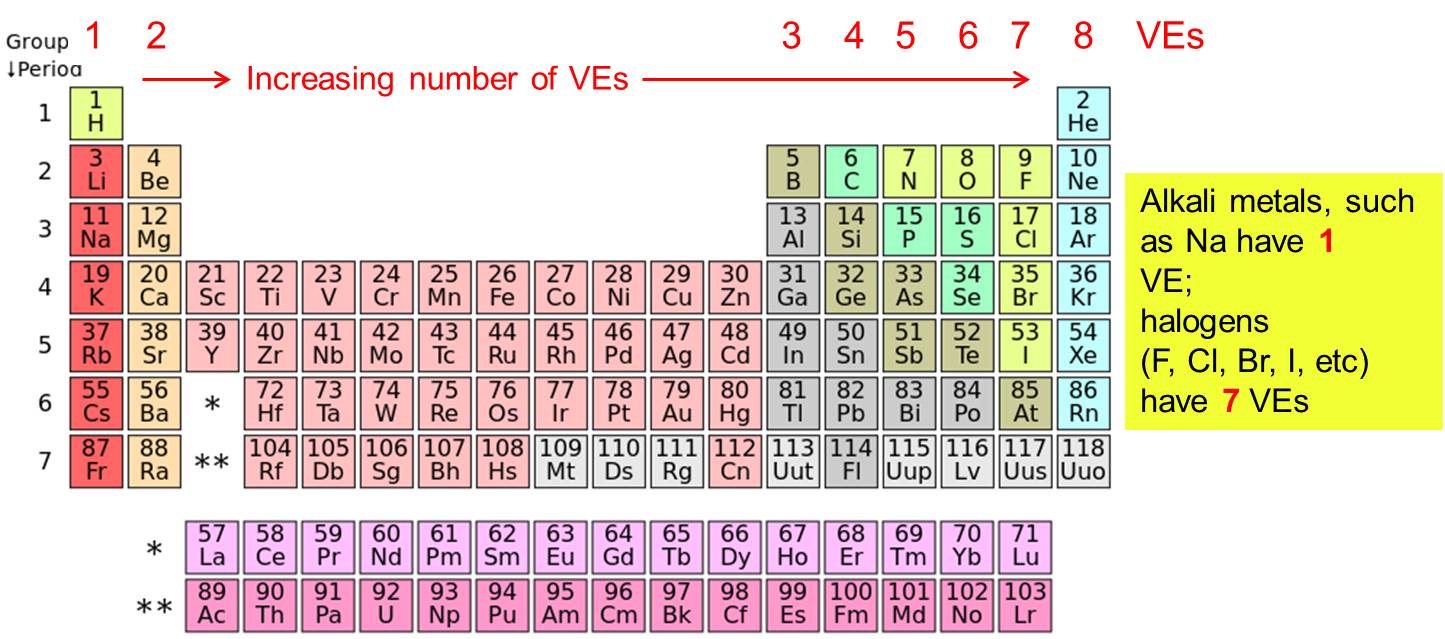
Valence electron
Electrons of the atoms outer shell that participate in the formation of a chemical bond.
Van Deemter equation
An equation used to explain band broadening in chromatography.
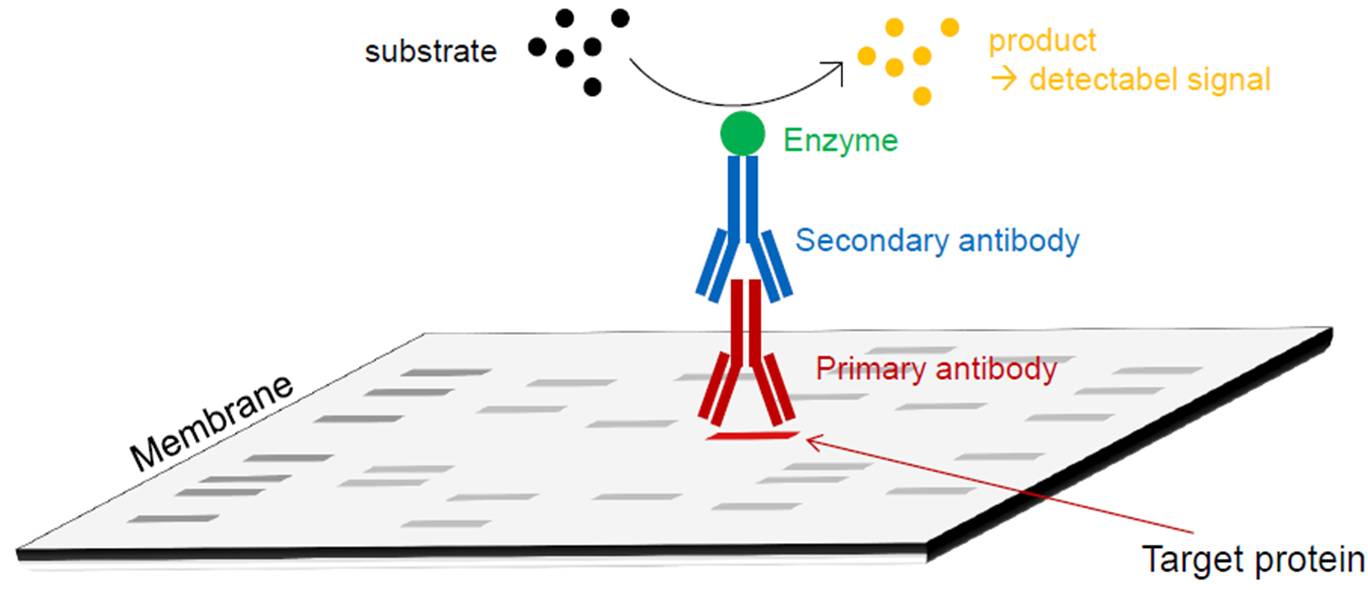
Western Blot
Method consists of a gel electrophoretic separation of proteins followed by the blotting of separated proteins onto a membrane. An antibody is added which specifically binds the target protein. A second antibody, on which a reporter enzyme is coupled, binds the first antibody. A detectable signal is generated by the enzymatic degradation of an added substrate and the generation of the respective product.